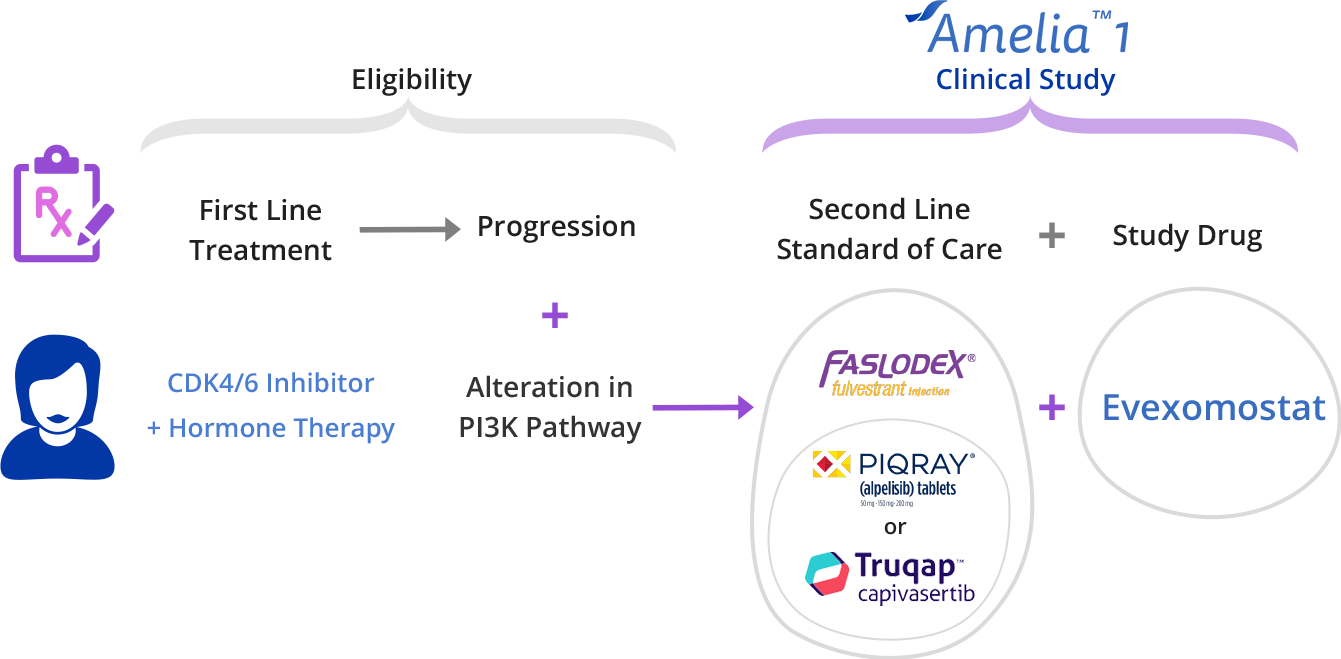
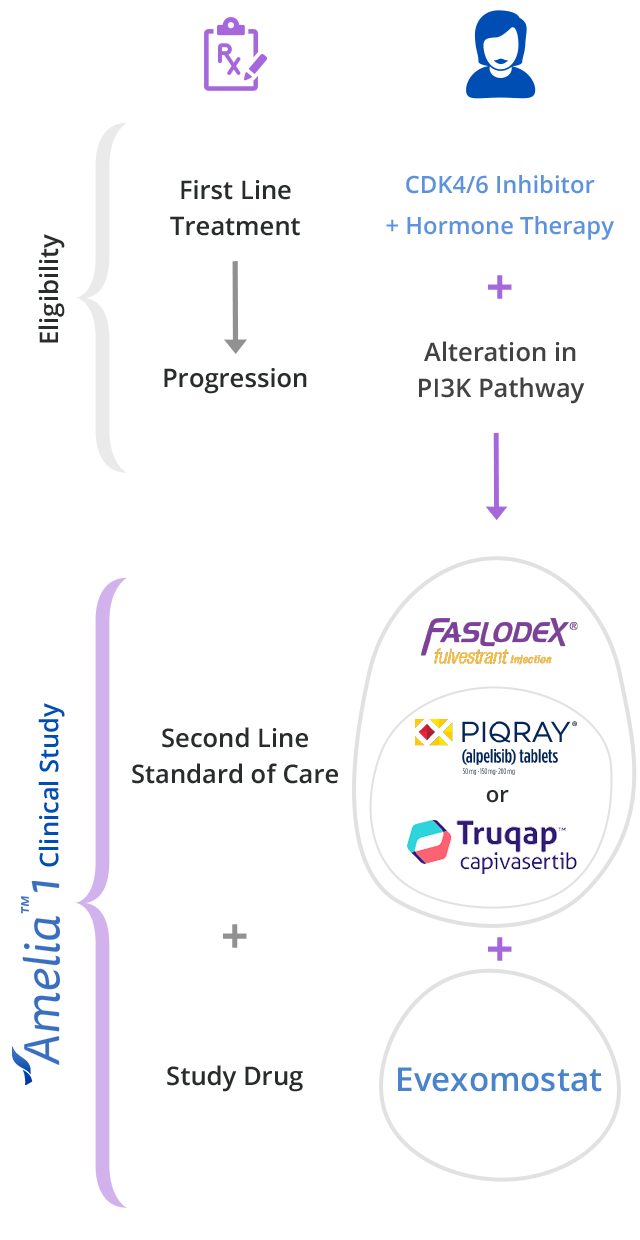
The Amelia-1 Clinical Trial is enrolling now for breast cancer survivors with a PI3K pathway alteration.
The Amelia-1 clinical trial is testing a new, experimental cancer treatment called Evexomostat to see if it improves clinical outcomes (better safety and efficacy)
when combined with either Piqray® or Truqap® and Faslodex®.
Hope is on the way!

Evexomostat May Improve Insulin Sensitivity to Improve Your Treatment
The Amelia-1 clinical trial is testing whether adding evexomostat (SDX-7320) to either Truqap or Piqray plus Faslodex will help reduce the side effects of Truqap or Piqray and control your cancer longer.
● Blood sugar spikes are a potentially serious side effect when taking drugs from the PI3K/AKT drug class, including Truqap and Piqray.
● High fasting insulin, either caused by high blood glucose (sugar) or due to insulin resistance, can lead to disease recurrence and more aggressive disease.
● Evexomostat is an experimental drug being developed for breast cancer patients like you that have an alteration in the PI3K/Akt pathway. Controlling high glucose could help improve clinical outcomes.
Clinical Rationale for the Amelia-1 Trial
✔ In a Phase 1 clinical trial in late-stage cancer patients, evexomostat significantly improved insulin sensitivity in patients with insulin resistance.
✔ The mechanism of action of evexomostat (MetAP2 inhibition) has been clinically shown to help improve patients’ insulin sensitivity and also provide anti-tumor activity.
✔ In animal models of breast cancer with the PIK3CA gene mutation, evexomostat combined with either Truqap or Piqray worked synergistically to shrink tumors.
✔ In normal, healthy animals, evexomostat significantly lowered the glucose spikes caused by either Truqap or Piqray.
**All clinical trials come with risks. Have your oncologist explain the risks to you before considering joining the Amelia-1 Trial.

Who Can Join?
Amelia-1 Enrollment Criteria
Patients that meet the following basic criteria may be eligible to join the study:
- HR+ (estrogen or progesterone), Her2-, metastatic breast cancer
- PI3K pathway alteration: PIK3CA or AKT1 gene mutation, PTEN loss
- Previously treated with a CDK 4/6 inhibitor (e.g., Kisqali® or Ibrance®) in 1st line setting
4. About to start 2nd line treatment for metastatic disease
⮚ Certain patients may be at increased risk for hyperglycemia (insulin resistant, high BMI).
⮚ There are other, more technical criteria that your health care provider or treating oncologist will review to be sure you qualify for the clinical study.
If you meet these basic criteria, you might benefit from this clinical study.
The Amelia-1 breast cancer clinical trial will enroll about 25-30 patients for each group initially to test the safety and efficacy for each of the three-drug combinations
(Evexomostat, Faslodex plus either Truqap or Piqray).
Additional patients may be added later.

Amelia-1 Breast Cancer Clinical Trial Locations
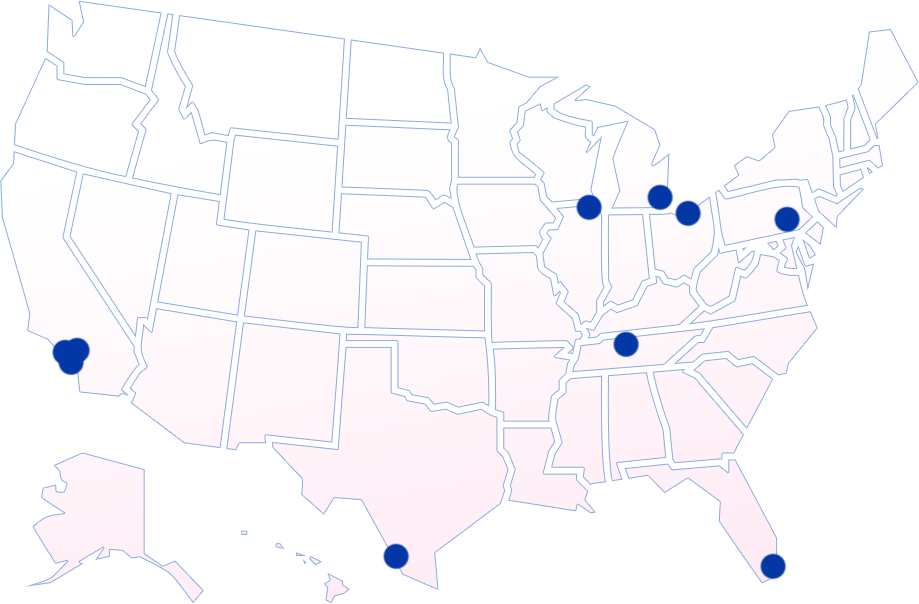
To enroll, please have your oncologists contact one of our open
trial locations.
Note - additional study sites will be added as they complete qualification.
California Locations:
Loma Linda University Cancer Center
11175 Campus Street, CSP 11005,
Loma Linda, CA 92350
Contact: Lorena Garcia, CRC
(909) 651-5612
PI: Gayathri Nagaraj, M.D
Hoag Memorial Hospital Presbyterian
One Hoag Drive, Building 39 2nd floor, Newport Beach, CA 92663
Contact: Jericho Rabago, BSN, RN
(949) 764-6796
PI: Chaitali Nangia, M.D
Sharp Memorial Hospital
7901 Frost Street
San Diego, CA 92123
Contact: Galen Steinhoff
PI: Kristen Rice, M.D
Florida Locations:
Miami Cancer Institute
1228 S. Pine Island Rd., Suite 410,
Plantation, FL 33324
Contact: Elaine Hernandez, Clinical Research Coordinator
PI: Reshma Mahtani, M.D
Miami Cancer Institute
8900 North Kendall Drive,
Miami, FL 33176
Contact: Elaine Hernandez, Clinical Research Coordinator
PI: Reshma Mahtani, M.D
Illinois Locations:
Hope and Healing Cancer Services
950 N. York Rd., Suite 201A,
Hinsdale, IL 60521
Contact: Praneetha Achanta
O: 630 560-0121 x106
F: 630-556-8014
PI: Srilata Gundala, M.D
Michigan Locations:
Trinity Health/St. Joseph Mercy
5301 East Huron River Drive,
Ann Arbor, MI 48106
Contact: Trinity Drews, BSN, OCN
tricia.drews@trinity-health.org
PI: Tareq Al Baghdadi
Ohio Locations:
Cleveland Clinic
9500 Euclid Avenue, CA5,
Cleveland, OH 44195
Contact: Donicka (Donnie) Wilson
PI: Azka Ali, M.D
Aultman Hospital
2600 Sixth Street Southwest,
Canton, OH 44710
Contact: Kathleen Collins, RN, BSN
PI: Raza Khan, M.D
Pennsylvania Locations:
Milton S. Hershey Medical Center
Penn State Cancer Institute
500 University Drive, Suite T2200,
Hershey, PA 17033
Contact: Cindy Brown
cbrown18@pennstatehealth.psu.edu
PI: Monali Vasekar, M.D
Tennessee Locations:
Vanderbilt Ingram Cancer Center
2220 Pierce Avenue, PRB 777,
Nashville, TN 37232
Contact: (800) 811-8484
PI: Brent Rexer, M.D PhD
Vanderbilt Ingram Cancer Center
719 Thompson Lane, Suite 25000,
Nashville, TN 37204
Contact: (800) 811-8484
PI: Brent Rexer, M.D PhD
You've got questions? We've got answers.
VIDEO - Metabo-Oncology: The Critical Role Dysregulated Metabolic Hormones Play in Cancer Progression
Dr. Neil Iyengar, Memorial Sloan Kettering Cancer Center in New York City, explains the impacts of obesity/pre-diabetes on breast cancer progression and patient clinical outcomes in this video:
Click to View the Video Here >>